中国生物技术2025年美国临床肿瘤学会(ASCO)摘要解读

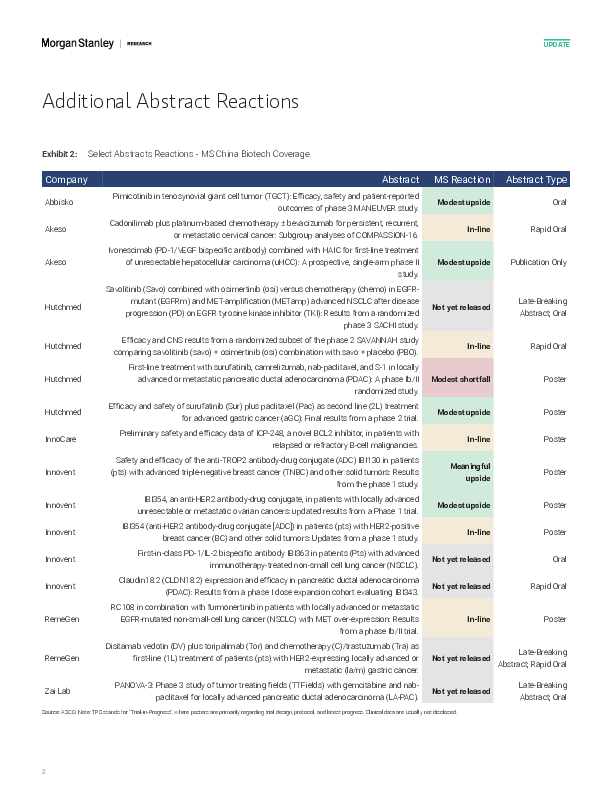



M UpdateChina Healthcare | Asia PacificChina Biotech: 2025 ASCO Abstract Reactions Morgan Stanley Asia Limited+Jack LinEquity Analyst Jack.Lin@morganstanley.com +852 3963-3746 China HealthcareAsia PacificIndustry ViewAttractiveFor detailed data comparison, see China Healthcare: China Biotech: AACR Takeaways, Out-licensing Update, and ASCO Preview. See inside for additional abstract reactions for MS China Biotech coverage. We share our initial thoughts on the ASCO abstracts, as well as expectations for the main event over May 30-Jun 3. DB-1311 in 3L CRPC - Median rPFS (n=57) continued to demonstrate best-in-class potential at 8.3m (vs. 7.2m previously) with the upper end of CI still not reached at 5.7 months of follow-up (link). Blended Gr3+ TRAE of 40% appears slightly higher than previously reported, and we await breakdown between 6mg/9mg cohorts.DB-1310 in pre-t
相关推荐
-
甲子光年2025年DeepSeeK开启AI算法变革元年报告16页

 2025-05-13 19957
2025-05-13 19957 -
少年商学院2025年DeepSeek中小学生使用手册81页

 2025-05-13 19840
2025-05-13 19840 -
英普利集团2025企业出海白皮书中东篇精编版39页

 2025-05-14 19543
2025-05-14 19543 -
曼昆律所2024年Web3.0区块链项目出海法律白皮书71页

 2025-05-14 18537
2025-05-14 18537 -
火山引擎2024火山引擎视频云实践精选集224页

 2025-05-15 18943
2025-05-15 18943 -
2025Q1中国企业创投[CVC]发展报告

 2025-06-05 311
2025-06-05 311 -
英文定价还是恐慌?商业房地产市场与气候变化

 2025-06-05 603
2025-06-05 603 -
对全球风险的预测敏感性:BVAR分析(英)2025

 2025-06-11 632
2025-06-11 632 -
超威驱蚊项目整合营销结案

 2025-06-13 954
2025-06-13 954 -
IT服务职位列表——2025年5月

 2025-07-11 397
2025-07-11 397
相关内容
-

甲子光年2025年DeepSeeK开启AI算法变革元年报告16页
分类:机构报告
时间:2025-05-13
标签:
格式:PDF
-

少年商学院2025年DeepSeek中小学生使用手册81页
分类:机构报告
时间:2025-05-13
标签:
格式:PDF
-

英普利集团2025企业出海白皮书中东篇精编版39页
分类:机构报告
时间:2025-05-14
标签:
格式:PDF
-

火山引擎2024火山引擎视频云实践精选集224页
分类:机构报告
时间:2025-05-15
标签:
格式:PDF
-

曼昆律所2024年Web3.0区块链项目出海法律白皮书71页
分类:机构报告
时间:2025-05-14
标签:
格式:PDF
-

中国购车用户家庭存款洞察报告 (2025版)
分类:
时间:2025-07-11
标签:
格式:PDF
-

2025应届生画像白皮书——以AI智略人才模型聚焦应届生新质人才潜能
分类:
时间:2025-07-11
标签:
格式:PDF
-
数据洞察:中国自由流动性与MSCI中国同比变化
分类:机构报告
时间:2025-06-08
标签:
格式:PDF
-

2025年KOC达人推广行业白皮书
分类:
时间:2025-07-11
标签:
格式:PDF
-

2025中国低空经济市场现状报告
分类:
时间:2025-07-11
标签:
格式:PDF





