为您的骨科器械选择合适的包装系统

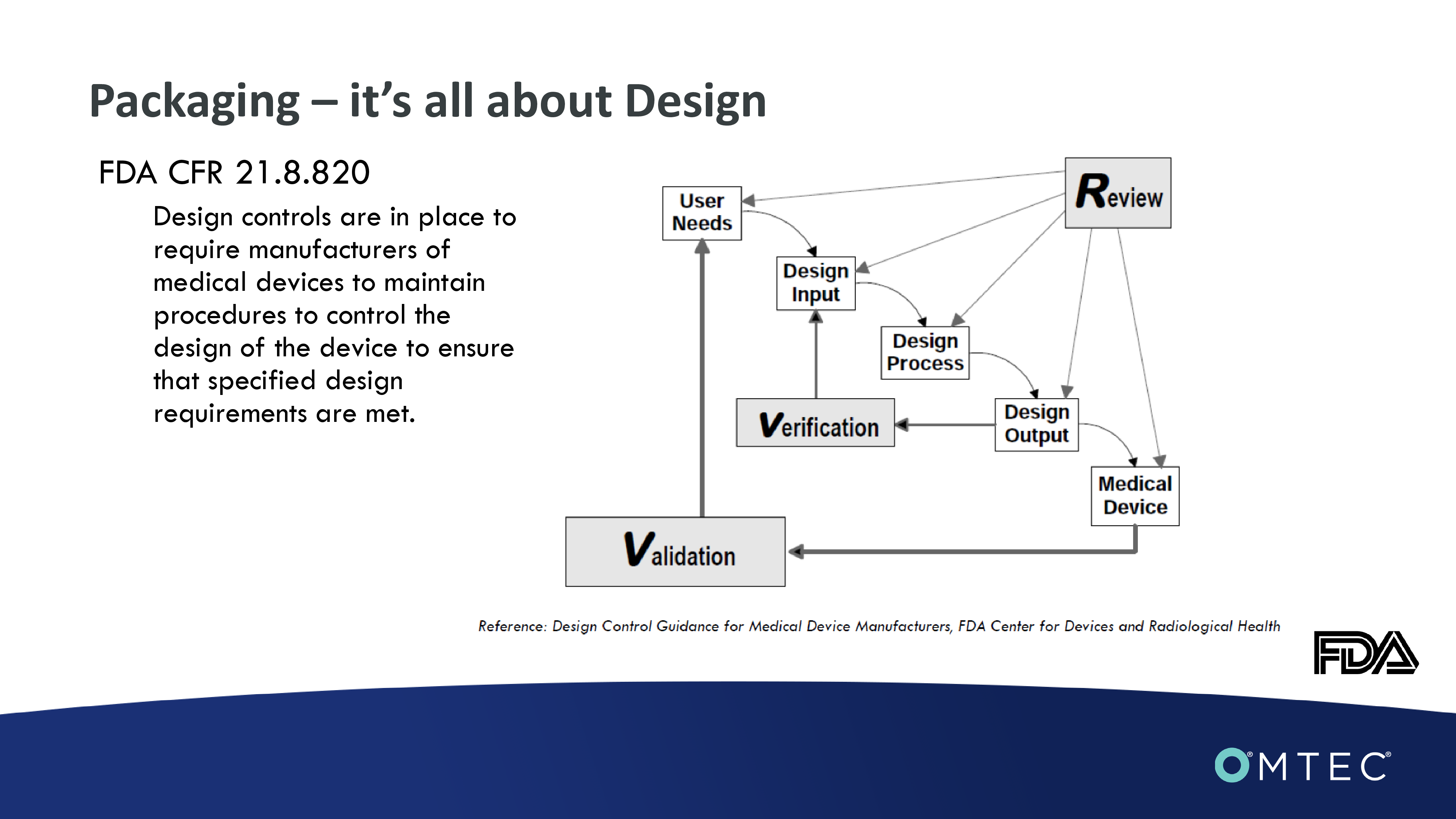


Sponsored byWendy Mach, Canyon LabsPick the Right Packaging System for Your Orthopedic DeviceAgenda•Design Input•Design Output•Design Review•Design Verification•Design Validation•Packaging Failures Packaging – it’s all about DesignFDA CFR 21.8.820Design controls are in place to require manufacturers of medical devices to maintain procedures to control the design of the device to ensure that specified design requirements are met. Design Input820.3(f) “Design input means the physical and performance requirements of a device that are used as a basis for device design.”Understand your user needs - voice of the customer•Package designs must be easy to open and intuitive. Systems should be transparent, allowing for quick recognition.•Visual cues and good thermoformed designs that will both protect and display items in a manner that supports the surgical team.•Shippers must ensure outer package integrity and durability.Listen for competitor complaintsDesign Input - Device protectionConsider i
相关推荐
-
鼎帷咨询2025年DeepSeek战略创新分析报告-围绕DeepSeek尖刀点加速打造AI产业刀锋链39页

 2025-05-13 19940
2025-05-13 19940 -
新战略咨询2024移动机器人AGV_AMR专用激光雷达产品发展蓝皮书31页

 2025-05-15 19947
2025-05-15 19947 -
甲子光年2025年DeepSeeK开启AI算法变革元年报告16页

 2025-05-13 19955
2025-05-13 19955 -
CyberRobo2024全球人形机器人产品数据库报告-人形机器人洞察研究BTIResearch99页

 2025-05-15 17948
2025-05-15 17948 -
少年商学院2025年DeepSeek中小学生使用手册81页

 2025-05-13 19839
2025-05-13 19839 -
英普利集团2025企业出海白皮书中东篇精编版39页

 2025-05-14 19541
2025-05-14 19541 -
曼昆律所2024年Web3.0区块链项目出海法律白皮书71页

 2025-05-14 18533
2025-05-14 18533 -
火山引擎2024火山引擎视频云实践精选集224页

 2025-05-15 18939
2025-05-15 18939 -
2025年无人机生态系统发展计划报告(英文版)-世界银行

 2025-06-05 472
2025-06-05 472 -
2025Q1中国企业创投[CVC]发展报告

 2025-06-05 307
2025-06-05 307
相关内容
-

甲子光年2025年DeepSeeK开启AI算法变革元年报告16页
分类:机构报告
时间:2025-05-13
标签:
格式:PDF
-

新战略咨询2024移动机器人AGV_AMR专用激光雷达产品发展蓝皮书31页
分类:机构报告
时间:2025-05-15
标签:
格式:PDF
-

鼎帷咨询2025年DeepSeek战略创新分析报告-围绕DeepSeek尖刀点加速打造AI产业刀锋链39页
分类:机构报告
时间:2025-05-13
标签:
格式:PDF
-

少年商学院2025年DeepSeek中小学生使用手册81页
分类:机构报告
时间:2025-05-13
标签:
格式:PDF
-

英普利集团2025企业出海白皮书中东篇精编版39页
分类:机构报告
时间:2025-05-14
标签:
格式:PDF
-

火山引擎2024火山引擎视频云实践精选集224页
分类:机构报告
时间:2025-05-15
标签:
格式:PDF
-

曼昆律所2024年Web3.0区块链项目出海法律白皮书71页
分类:机构报告
时间:2025-05-14
标签:
格式:PDF
-

CyberRobo2024全球人形机器人产品数据库报告-人形机器人洞察研究BTIResearch99页
分类:机构报告
时间:2025-05-15
标签:
格式:PDF
-

中国购车用户家庭存款洞察报告 (2025版)
分类:
时间:2025-07-11
标签:
格式:PDF
-

2025中国低空经济市场现状报告
分类:
时间:2025-07-11
标签:
格式:PDF





