根据ISO标准进行工艺验证的重要性


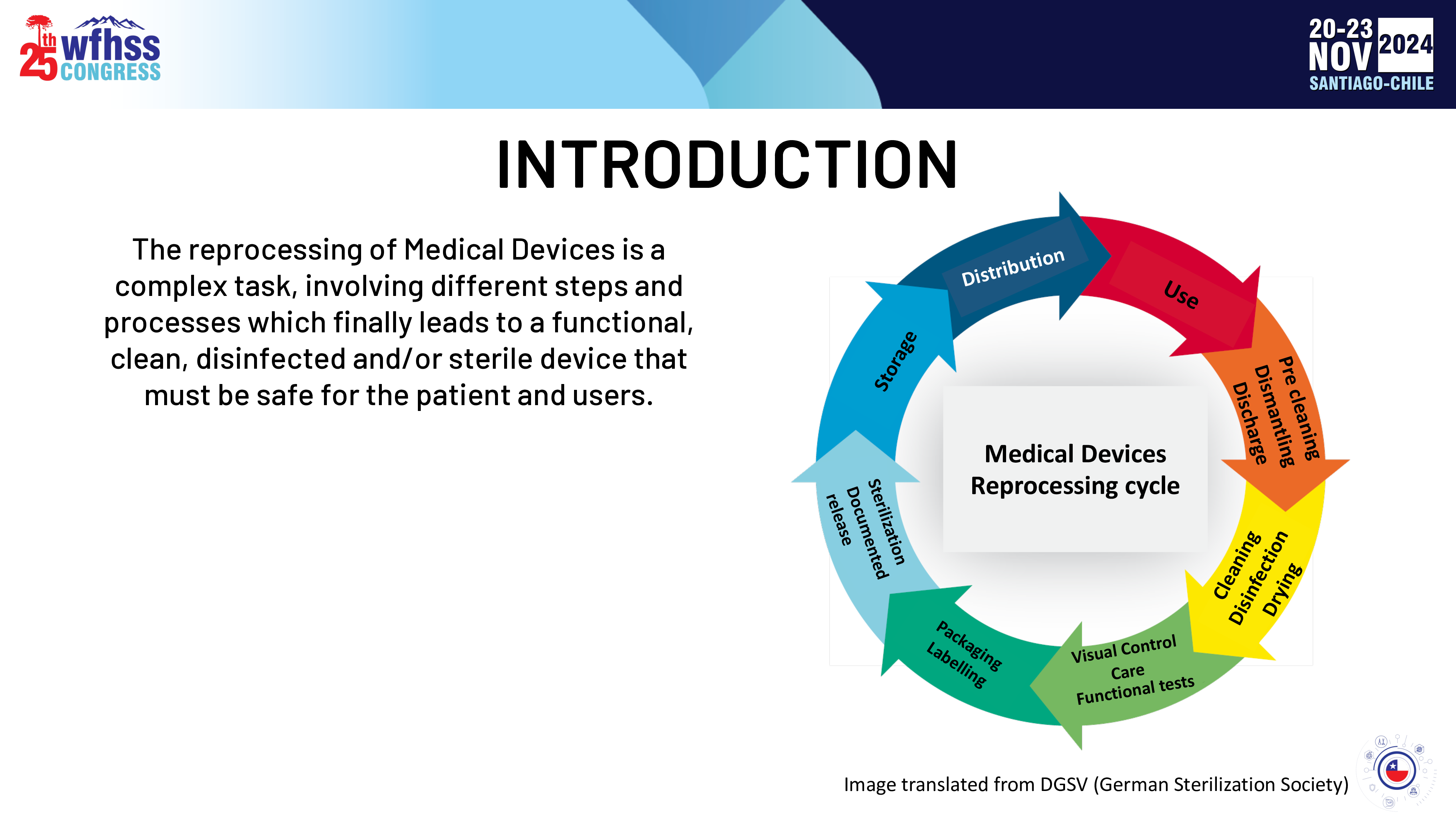
Importance of process validation according to ISO Name: MatíasPilasiPendásAffiliation: INDE / P&ECONTENTINTRODUCTIONBACKGROUNDWHY IS VALIDATION NEEDED?ISO STANDARDS FOR VALIDATIONHOW IT IS CARRIED OUTCONCLUSIONSINTRODUCTIONUseThe reprocessing of Medical Devices is a complex task, involving different steps and processes which finally leads to a functional, clean, disinfected and/or sterile device that must be safe for the patient and users. CleaningDisinfectionDryingPackagingLabellingVisual ControlCareFunctional testsSterilizationDocumentedrelease StorageDistributionMedical Devices Reprocessing cycleImage translated from DGSV (German Sterilization Society)Pre cleaningDismantlingDischargeINTRODUCTIONAccording to good practices from an Infection Control and Safe Surgeries perspective, Medical Devices that will come in direct contact with sterile areas of the human body must be STERILEINTRODUCTIONHow do me measure the sterility of Medical Devices?It´s not possible……INTRODUCTIONProblemInstr
相关推荐
-
鼎帷咨询2025年DeepSeek战略创新分析报告-围绕DeepSeek尖刀点加速打造AI产业刀锋链39页

 2025-05-13 19936
2025-05-13 19936 -
新战略咨询2024移动机器人AGV_AMR专用激光雷达产品发展蓝皮书31页

 2025-05-15 19943
2025-05-15 19943 -
甲子光年2025年DeepSeeK开启AI算法变革元年报告16页

 2025-05-13 19950
2025-05-13 19950 -
CyberRobo2024全球人形机器人产品数据库报告-人形机器人洞察研究BTIResearch99页

 2025-05-15 17939
2025-05-15 17939 -
少年商学院2025年DeepSeek中小学生使用手册81页

 2025-05-13 19833
2025-05-13 19833 -
英普利集团2025企业出海白皮书中东篇精编版39页

 2025-05-14 19537
2025-05-14 19537 -
曼昆律所2024年Web3.0区块链项目出海法律白皮书71页

 2025-05-14 18531
2025-05-14 18531 -
火山引擎2024火山引擎视频云实践精选集224页

 2025-05-15 18933
2025-05-15 18933 -
2025年无人机生态系统发展计划报告(英文版)-世界银行

 2025-06-05 465
2025-06-05 465 -
2025Q1中国企业创投[CVC]发展报告

 2025-06-05 301
2025-06-05 301
相关内容
-

甲子光年2025年DeepSeeK开启AI算法变革元年报告16页
分类:机构报告
时间:2025-05-13
标签:
格式:PDF
-

新战略咨询2024移动机器人AGV_AMR专用激光雷达产品发展蓝皮书31页
分类:机构报告
时间:2025-05-15
标签:
格式:PDF
-

鼎帷咨询2025年DeepSeek战略创新分析报告-围绕DeepSeek尖刀点加速打造AI产业刀锋链39页
分类:机构报告
时间:2025-05-13
标签:
格式:PDF
-

少年商学院2025年DeepSeek中小学生使用手册81页
分类:机构报告
时间:2025-05-13
标签:
格式:PDF
-

英普利集团2025企业出海白皮书中东篇精编版39页
分类:机构报告
时间:2025-05-14
标签:
格式:PDF
-

火山引擎2024火山引擎视频云实践精选集224页
分类:机构报告
时间:2025-05-15
标签:
格式:PDF
-

曼昆律所2024年Web3.0区块链项目出海法律白皮书71页
分类:机构报告
时间:2025-05-14
标签:
格式:PDF
-

CyberRobo2024全球人形机器人产品数据库报告-人形机器人洞察研究BTIResearch99页
分类:机构报告
时间:2025-05-15
标签:
格式:PDF
-

2025泡泡玛特POP MART品牌手册
分类:
时间:2025-06-21
标签:
格式:PDF
-

利用人工智能技术全面应对电子邮件威胁
分类:
时间:2025-06-21
标签:
格式:PDF





